Greenhouse gases cannot physically cause observed global warming
The World Is Warming
All four major analyses of Earth’s average surface temperatures (NASA, NOAA, Hadcrut4, and Berkeley Earth) document global warming since 1950 of approximately 0.9 degrees Celsius (1.6 degrees Fahrenheit). There was very little change in temperature from 1950 to 1970. The world warmed 0.6 degrees from 1970 to 1998. There was very little change in temperature from 1998 through 2013. Then the world warmed an additional 0.3 degrees from 2013 to 2016, making 2016 the hottest year on record. Most climate scientists are convinced, based on greenhouse-warming theory, that this warming is caused primarily by increased burning of fossil fuels, causing increasing emissions of greenhouse gases, that are absorbing increasing amounts of infrared radiation emitted by Earth. Annual, average concentrations of carbon dioxide measured on Mauna Loa, Hawaii, have increased from 316 ppm in 1959 to 411 ppm in 2019 (30%). Most climate scientists are convinced that we must decrease burning of fossil fuels substantially and promptly in order to prevent dangerous overheating of Earth during the next few decades.
Why the Physics of Greenhouse-Warming Theory Appears to Be Mistaken
Greenhouse-warming theory, according to the Intergovernmental Panel on Climate Change, posits that greenhouse gases, primarily water vapor (H2O), carbon dioxide (CO2), nitrous oxide (N2O), methane (CH4) and ozone (O3)
“absorb terrestrial radiation emitted by Earth’s surface and elsewhere in the atmosphere. These substances emit infrared radiation in all directions, but, everything else being equal, the net amount emitted to space is normally less than would have been emitted in the absence of these absorbers. … An increase in the concentration of greenhouse gases increases the magnitude of this effect.”
Greenhouse-warming theory is thus based on the fundamental assumption described by Joseph Fourier in 1822 (Page 3) that the average temperature at Earth’s surface will increase if the net amount of terrestrial radiation reaching space becomes less than the net amount of solar radiation reaching Earth, often explained as the Earth-atmosphere energy balance, Earth’s energy budget, Earth’s radiation budget, tracking Earth’s energy, or Earth’s energy balance).
Since 1798, physicists have thought of heat as thermal energy in transfer—a flux through some surface measured in watts per square meter, where watts are the number of joules of energy passing through the surface each second. Climate scientists calculate radiative forcings, which are the net changes in flux (downward minus upward) caused by changes in concentrations in the atmosphere of each type of greenhouse gas, dust, black carbon soot, smoke from biomass burning, aerosols, volcanic aerosols, contrails, and such, or caused by any changes in radiation from Sun. They add these radiative forcings together to estimate changes in temperature.
Thus greenhouse-warming theory is based on the assumption that (1) radiative energy can be quantified by a single number of watts per square meter, (2) the assumption that these radiative forcings can be added together, and (3) the assumption that Earth’s surface temperature is proportional to the sum of all of these radiative forcings. A fundamentally new understanding of the physics of thermal energy and the physics of heat, described below, shows that all three assumptions are mistaken. There are other serious problems: (4) greenhouse gases absorb only a small part of the radiation emitted by Earth, (5) they can only reradiate what they absorb, (6) they do not reradiate in every direction as assumed, (7) they make up only a tiny part of the gases in the atmosphere, and (8) they have been shown by experiment not to cause significant warming. (9) The thermal effects of radiation are not about amount of radiation absorbed, as currently assumed, they are about the temperature of the emitting body and the difference in temperature between the emitting and the absorbing bodies as described below.
The thermal effects of radiation are not about amount of radiation absorbed, as currently assumed, they are about the temperature of the emitting body and the difference in temperature between the emitting and the absorbing bodies.
Heat is, in concept, what a body of matter must absorb to get warmer and emit to get cooler. Defining heat as thermal energy in transfer completely sidesteps the issue of what thermal energy is physically in matter and in radiation. Physics is supposed to be about what is physically happening in Nature. What physically is thermal energy? What physically is heat? Once we can answer these questions, then we can observe how, physically, thermal energy and heat flow through matter, through air, and through space?
What Physically Is Thermal Radiation?
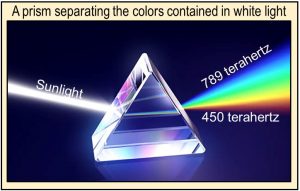 In 1672, Isaac Newton showed, using a prism and lenses, that sunlight is made up of a broad spectrum of colors and that these colors physically coexist within sunlight—they are displayed by but not created by the prism. Newton also showed that by using a second prism, he could combine the colors back into white light.
In 1672, Isaac Newton showed, using a prism and lenses, that sunlight is made up of a broad spectrum of colors and that these colors physically coexist within sunlight—they are displayed by but not created by the prism. Newton also showed that by using a second prism, he could combine the colors back into white light.
We observe that these colors do not interact with each other in any way in air and space—only when in the immediate presence of matter. We refer to these colors as the visible spectrum extending from red through violet, but they clearly form a continuous spectrum or continuum meaning a continuous sequence of shades of color in which adjacent colors are not perceptibly different from each other, although the extremes are quite distinct. The human eye can detect about ten million distinct colors.
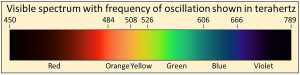 We physically measure visible light as containing all frequencies of oscillation ranging from 450 to 789 terahertz, where one terahertz is one-trillion cycles per second (1012 cycles per second). We also observe that the visible spectrum is but a very small part of a much wider continuum that we call electromagnetic radiation
We physically measure visible light as containing all frequencies of oscillation ranging from 450 to 789 terahertz, where one terahertz is one-trillion cycles per second (1012 cycles per second). We also observe that the visible spectrum is but a very small part of a much wider continuum that we call electromagnetic radiation 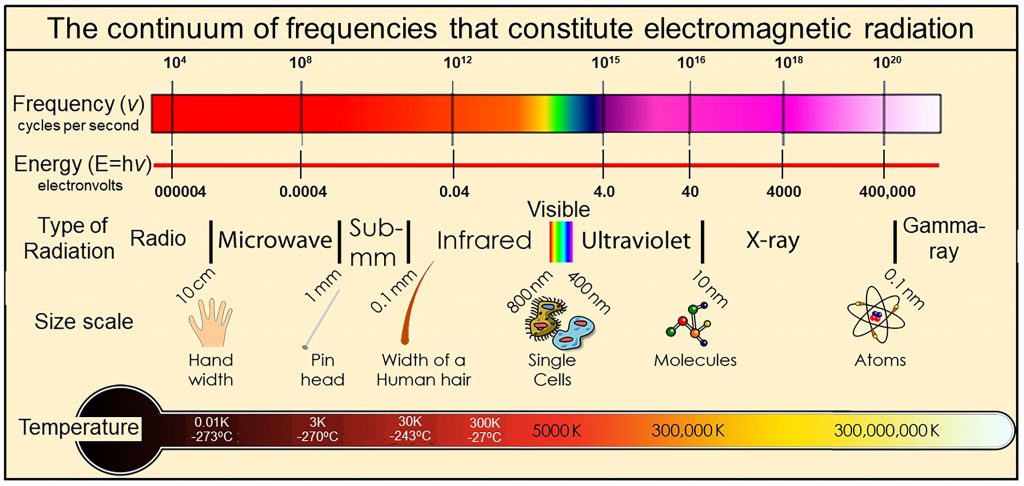 with frequencies extending over more than 20 orders of magnitude from extremely low frequency radio signals in cycles per second to microwave, infrared, visible, ultraviolet, X-rays, to gamma rays with frequencies of more than 100 million, million, million cycles per second (1020 cycles per second). Thermal radiation is a portion of this continuum of electromagnetic radiation radiated by a body of matter as a result of the body’s temperature—the hotter the body, shown here at the bottom as Temperature, the higher the radiated frequencies of oscillation with significant amplitudes of oscillation.
with frequencies extending over more than 20 orders of magnitude from extremely low frequency radio signals in cycles per second to microwave, infrared, visible, ultraviolet, X-rays, to gamma rays with frequencies of more than 100 million, million, million cycles per second (1020 cycles per second). Thermal radiation is a portion of this continuum of electromagnetic radiation radiated by a body of matter as a result of the body’s temperature—the hotter the body, shown here at the bottom as Temperature, the higher the radiated frequencies of oscillation with significant amplitudes of oscillation.
We observe that electromagnetic radiation has two physical properties: 1) frequency of oscillation, which is color in the visible part of the continuum, and 2) amplitude of oscillation, which we perceive as intensity or brightness at each frequency.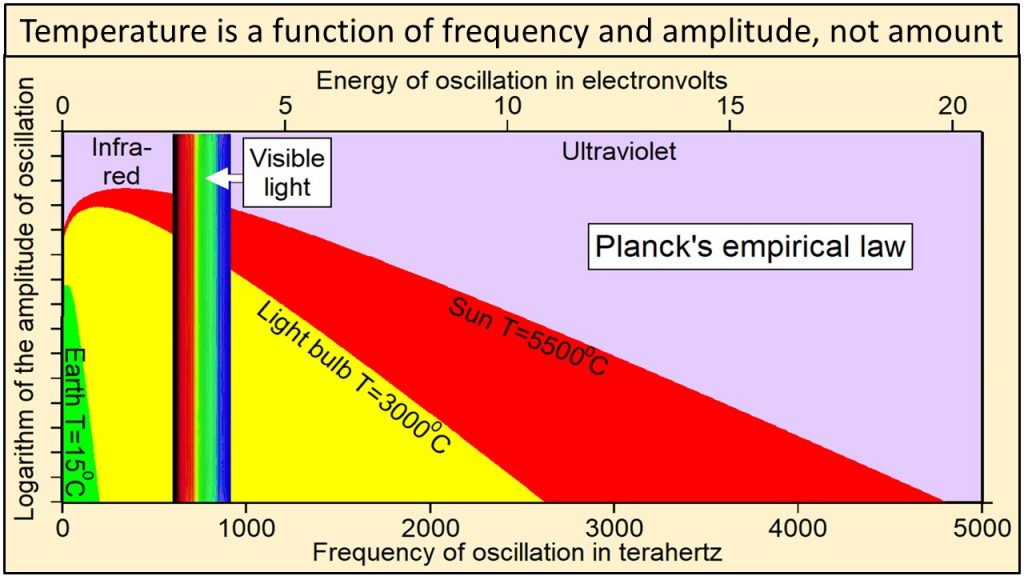 In 1900, Max Planck, one of the fathers of modern physics, derived an equation by trial and error that has become known as Planck’s empirical law. Planck’s empirical law is not based on theory, although several derivations have been proposed. It was formulated solely to calculate correctly the intensities at each frequency observed during extensive direct observations of Nature. Planck’s empirical law calculates the observed intensity or amplitude of oscillation at each frequency of oscillation for radiation emitted by a black body of matter at a specific temperature and at thermal equilibrium. A black body is simply a perfect absorber and emitter of all frequencies of radiation.
In 1900, Max Planck, one of the fathers of modern physics, derived an equation by trial and error that has become known as Planck’s empirical law. Planck’s empirical law is not based on theory, although several derivations have been proposed. It was formulated solely to calculate correctly the intensities at each frequency observed during extensive direct observations of Nature. Planck’s empirical law calculates the observed intensity or amplitude of oscillation at each frequency of oscillation for radiation emitted by a black body of matter at a specific temperature and at thermal equilibrium. A black body is simply a perfect absorber and emitter of all frequencies of radiation.
Thermal radiation from Earth, at a temperature of 15 oC, consists of the narrow continuum of frequencies of oscillation shown in green in this plot of Planck’s empirical law. Thermal radiation from the tungsten filament of an incandescent light bulb at 3000 oC consists of a broader continuum of frequencies shown in yellow and green. Thermal radiation from Sun at 5500 oC consists of a much broader continuum of frequencies shown in red, yellow and green.
Note in this plot of Planck’s empirical law that the higher the temperature, 1) the broader the continuum of frequencies, 2) the higher the amplitude of oscillation at each and every frequency, and 3) the higher the frequencies of oscillation that are oscillating with the largest amplitudes of oscillation. Radiation from Sun shown in red, yellow, and green clearly contains much higher frequencies and amplitudes of oscillation than radiation from Earth shown in green. Planck’s empirical law shows unequivocally that the physical properties of radiation are a function of the temperature of the body emitting the radiation.
Planck’s empirical law shows unequivocally that the physical properties of radiation are a function of the temperature of the body emitting the radiation.
Heat, defined in concept as that which must be absorbed by solid matter to increase its temperature, is similarly a broad continuum of frequencies of oscillation and corresponding amplitudes of oscillation. For example, the broad continuum of heat that Earth, with a temperature of 15 oC, must absorb to reach a temperature of 3000 oC is shown by the continuum of values within the yellow-shaded area in this plot of Planck’s empirical law.
Heat is, therefore, a broad continuum of frequencies and amplitudes of oscillation that cannot be described by a single number of watts per square meter as currently assumed in physics and in greenhouse-warming theory. The physical properties of heat as described by Planck’s empirical law and the thermal effects of this heat are determined both by the temperature of the emitting body and, as we will see below, by the difference in temperature between the emitting body and the absorbing body. But what is oscillating?
Heat is a broad continuum of frequencies and amplitudes of oscillation that cannot be described by a single number of watts per square meter as currently assumed.
Oscillation of All the Bonds Holding Matter Together
We observe that when a radio transmitter applies a frequency of oscillation to its antenna, that specific frequency is transmitted through air and space where it can be received by any radio receiver tuned to resonate at that specific frequency and located at a reasonable distance within or very close to line of sight. We also observe that a very large number of these radio frequencies coexist within the electromagnetic continuum where they do not interact in air or space. Physicists and electrical engineers think of transmission of frequency as the result of the physical motion of electric charge on the surface of the antenna of a radio transmitter.
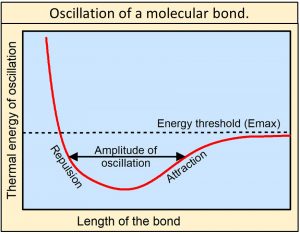 We also observe that the chemical bonds holding matter together are not rigid. They oscillate between electrodynamic forces of repulsion when the atoms get too close together and electrodynamic forces of attraction as the atoms move apart as approximated by the Morse potential shown in this figure, or by the more detailed Morse/Long-range potential. Oscillations of bonds are frictionless and therefore can last essentially forever. As the amplitude of oscillation increases, the energy of oscillation increases until the energy of oscillation reaches a threshold (Emax) equal to the energy holding the bond together. At this level of energy, the bond is essentially shaken apart—the bond is dissociated.
We also observe that the chemical bonds holding matter together are not rigid. They oscillate between electrodynamic forces of repulsion when the atoms get too close together and electrodynamic forces of attraction as the atoms move apart as approximated by the Morse potential shown in this figure, or by the more detailed Morse/Long-range potential. Oscillations of bonds are frictionless and therefore can last essentially forever. As the amplitude of oscillation increases, the energy of oscillation increases until the energy of oscillation reaches a threshold (Emax) equal to the energy holding the bond together. At this level of energy, the bond is essentially shaken apart—the bond is dissociated.
Thus, the frequencies and amplitudes of oscillation contained within thermal radiation and described by Planck’s empirical law must also be the frequencies and amplitudes of oscillation of molecular bonds on the surface of the emitting body that are transmitting the radiation. They must also exist below the surface of the radiating body, interacting via conduction if the body is at thermal equilibrium. In this way, Planck’s empirical law shows the broad continuum of frequencies and amplitudes of oscillation of all the bonds that must exist throughout a body of matter for that body to physically be at a specific temperature.
Planck’s empirical law shows the broad continuum of frequencies and amplitudes of oscillation of all the bonds that must exist throughout a body of matter for that body to physically be at a specific temperature.
Planck’s empirical law shows clearly that to increase the temperature of solid matter, you must increase the amplitude of oscillation at each and every frequency of oscillation and you must increase the frequency of oscillation that has the maximum amplitude of oscillation. The body, to be warmed, must absorb substantial amplitudes of oscillation primarily at the higher frequencies of oscillation. These higher amplitudes and frequencies of oscillation only occur in a body of matter that is hotter. We observe that amplitude of oscillation can only “flow” from a hotter to a cooler body of matter.
Thermal Energy is Similarly a Broad Continuum of Energies
To develop his law, Planck postulated that thermal energy (E) at the molecular-bond level, equals the Planck constant (h) times the frequency of oscillation (ν, the Greek letter nu). This simple equation, E=hν, says that the level of thermal energy (E) of oscillation of a single, frictionless, molecular-bond-scale oscillator is merely the frequency of oscillation of that particular oscillator times a scaling constant (h). Thus, energy of oscillation is the same physical thing as frequency of oscillation, and the Planck constant (h) is the number of electronvolts or joules of energy contained in a frequency of oscillation of one cycle per second.
E=hν says that energy of oscillation is the same physical thing as frequency of oscillation. The Planck constant is the number of joules of energy contained in a frequency of oscillation of one cycle per second.
Since radiant energy is a function of frequency of oscillation, E=hν must be plotted on an alternative x-axis shown at the top of the plot of Planck’s empirical law. Even though Planck postulated that energy equals a constant times frequency (E=hν) in order to write his law, he thought of this simple equation as “a purely formal assumption” stating that “I really did not give it much thought except that no matter what the cost, I must bring about a positive result.” Planck never stopped to think that E=hν means energy should be plotted on an alternative x-axis, not on the y-axis as widely thought at that time and still today. E=hν is now known as the Planck-Einstein relation and is essentially universally accepted as physically accurate.
E=hν also applies at the macroscopic level to the total thermal energy contained within radiation. In this case, frequency of oscillation (ν), plotted on the lower x-axis, is observed to be the broad continuous electromagnetic spectrum or continuum of values that all coexist. Similarly, energy (E), plotted on the upper x-axis, is observed to be the broad continuous spectrum or continuum of values of energy in units of joules or electronvolts that all coexist. Note from Planck’s empirical law that thermal energy is only a function of frequency of oscillation. Thermal energy is not a function of intensity, amplitude, or amount as currently assumed in physics and in greenhouse-warming theory.
Currently climate scientists plot energy on the y-axis and integrate as a function of frequency. It makes no physical sense to integrate as a function of frequencies, which means to add up frequencies. If you add red light to blue light, you end up with some red light and some blue light that coexist. There is no way in air and space that frequencies can be physically added together to equal a new frequency or to sum up energies to create a single number representing the total energy. Energy is a broad continuum of values.
The fundamental mistakes with greenhouse-warming theory are plotting energy on the y-axis of Planck’s empirical law and integrating as a function of frequency to get one number for the total amount of thermal energy flowing per second in units of watts per square meter. This method grossly overestimates the thermal effects of infrared energy.
The fundamental mistakes with greenhouse-warming theory are plotting energy on the y-axis of Planck’s empirical law and integrating as a function of frequency to get one number for the total amount of thermal energy flowing per second in units of watts per square meter. This method grossly overestimates the thermal effects of infrared energy.
There is a different value of energy and flux of energy at each and every frequency. The total energy of radiation, therefore, is the result of the simultaneous oscillation of all the bonds holding matter together—the co-existence of all frequencies and energies of oscillation. Thermal radiation is the result of a very, very large number of molecular-scale oscillators, all oscillating simultaneously on the surface of the radiating body. There is, physically, no such thing as a single total amount of thermal energy flowing per second in units of watts per square meter as currently assumed in physics and in greenhouse-warming theory. There is a level of energy at each frequency of oscillation, not a single total amount of energy as currently assumed. The variable closest in concept to amount in Planck’s empirical law is amplitude or intensity of oscillation.
Thermal radiation is the result of a very, very large number of molecular-scale oscillators, all oscillating simultaneously on the surface of the radiating body.
To summarize, Planck’s empirical law was derived by trial and error to describe accurately the observed physical properties of thermal radiation as a function of temperature, which are the frequencies of oscillation and their associated intensities or amplitudes of oscillation as a function of temperature. Planck’s empirical law shows clearly that what we perceive as temperature of matter is the result of a very broad spectrum or continuum of frequencies of oscillation of all the bonds holding matter together. Similarly, increased temperature is the result of increased amplitudes of oscillation at each and every frequency of oscillation, especially at higher frequencies.
Planck’s empirical law shows clearly that what we perceive as temperature of matter is the result of a very broad spectrum or continuum of frequencies of oscillation of all the bonds holding matter together.
For the temperature of a body of matter to increase by absorbing radiation, that radiation must come from a hotter body that is emitting radiation containing higher amplitudes of oscillation at each and every frequency of oscillation.
Earth Cannot Be Warmed by Its Own Radiation
Greenhouse-warming theory posits that average global surface temperatures rise when increasing concentrations of greenhouse gases in Earth’s atmosphere absorb increasing amounts of infrared radiation from Earth. In other words, the surface of Earth is warmed when Earth’s lower atmosphere absorbs thermal radiation emitted at Earth’s surface. This rise in temperature is estimated to be between 1.5 °C and 4.5 °C. when the atmospheric concentration of carbon dioxide is doubled.
But we observe clearly in Nature that no body of matter can be warmed in any way by absorbing its own radiation. Such warming is not physically possible. If it were possible, bodies of matter could, under the right circumstances, spontaneously heat up—something we all know does not happen. We would have an inexhaustible source of free energy—something too good to be true.
Imagine two bodies of solid matter positioned next to each other. Each at the same temperature. Each potentially absorbing identical radiation from the other. Neither can become hotter. Neither will get hotter, no matter how long you wait. Both bodies can lose heat to cooler surroundings, but neither can absorb heat from the other body as long as both bodies are at the same temperature.
The reason a body of matter cannot be heated by its own radiation is because its own radiation does not contain the higher amplitudes of oscillation at every frequency of oscillation that must be absorbed to physically increase the body’s temperature.
The reason a body of matter cannot be heated by its own radiation is because its own radiation does not contain the higher amplitudes of oscillation at every frequency of oscillation that must be absorbed to physically increase the body’s temperature.
Fourier and most climate scientists today would argue that greenhouse gases slow the cooling of Earth, which Sun, therefore, makes hotter. But Planck’s empirical law shows that the only way for Sun to make Earth hotter is when Earth absorbs higher than normal amplitudes of oscillation at higher frequencies of oscillation. This is precisely what is observed to happen when the ozone layer is depleted. Greater than normal amplitudes of oscillation of ultraviolet-B radiation are observed to pass through the depleted ozone layer, reaching Earth. Ultraviolet-B is the highest frequency, most energetic radiation to normally reach Earth’s surface. The average temperature of Earth’s surface is determined primarily by the optical thickness of the ozone layer. The less ozone in the ozone layer, the greater the amplitudes of oscillation of ultraviolet-B radiation reaching Earth, causing Earth to warm.
The average temperature of Earth’s surface is determined primarily by the optical thickness of the ozone layer. The less ozone in the ozone layer, the greater the amplitudes of oscillation of ultraviolet-B radiation reaching Earth, causing Earth to warm.
Earth Cannot Be Warmed by a Blanket of Greenhouse Gases
Some scientists propose that greenhouse gases act like a blanket surrounding Earth, keeping Earth approximately 33 oC warmer than expected for a planet at Earth’s distance from Sun. Blankets are well-known to slow the loss of thermal energy from a body of matter, but a blanket has no way to increase the amplitudes of oscillation at every frequency of oscillation. A blanket cannot be the source of new thermal energy required to increase the temperature of the body under the blanket, unless it is an electric blanket that adds thermal energy from somewhere else.
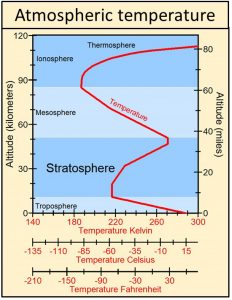 Earth’s blanket is observed to be the stratosphere, which does act like an electric blanket because it is heated by ultraviolet radiation from Sun, not by the body under the blanket, Earth. The stratosphere is the only part of the atmosphere below the thermosphere where temperatures increase with increasing altitude from an average of around -51 oC at the base of the stratosphere to an average of around ‑15 oC at the top of the stratosphere, approximately 36 degrees of warming.
Earth’s blanket is observed to be the stratosphere, which does act like an electric blanket because it is heated by ultraviolet radiation from Sun, not by the body under the blanket, Earth. The stratosphere is the only part of the atmosphere below the thermosphere where temperatures increase with increasing altitude from an average of around -51 oC at the base of the stratosphere to an average of around ‑15 oC at the top of the stratosphere, approximately 36 degrees of warming.
This temperature increase is caused by high-energy, solar, ultraviolet radiation dissociating any bond holding together gas molecules such as oxygen (O2), ozone (O3), or carbon dioxide (CO2). Upon dissociation, when the bond is broken, the pieces of a gas molecule fly apart at high velocity. Temperature of a gas is well-known to be proportional to the average kinetic energy of translation of all gas molecules, which for a single molecule is equal to one half its mass times its velocity squared. Thus, when a molecular bond is dissociated, all of the energy holding that bond together is converted instantaneously and completely into increased air temperature.
A similar example is found on Venus where the atmosphere is more than 96% carbon dioxide. It is dissociation of carbon dioxide by solar ultraviolet-C radiation that most likely causes the average surface temperature of Venus to be approximately 462 oC.
What is most surprising, given the importance of greenhouse gases in today’s politics, is that greenhouse gases absorbing infrared radiation have never been shown by experiment, a cornerstone of the scientific method, to cause any significant increase in air temperature as explained at JustProveCO2.com.
Greenhouse gases absorbing infrared radiation have never been shown by experiment to cause any significant increase in air temperature.
How Physically Does a Broad Continuum of Frequencies of Oscillation Flow Through Air and Space?
If you take two bodies of matter that are identical in every way except for temperature and connect them together so that heat can flow by radiation or by conduction, the resulting temperature, at thermal equilibrium, is observed to be the average of the two temperatures, not the sum of the two temperatures. Temperature is not additive—it is averative, a word I have coined to make this distinction. Additive is defined as of, relating to, or characterized by addition. Averative is defined as of, relating to, or characterized by averaging.
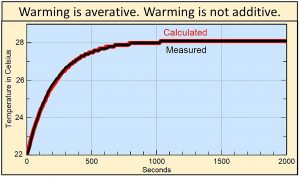 If you shine a light on a small black object, the rate of warming, the rate that heat flows, similarly decreases with decreasing difference in temperature forming an asymptotic curve shown by the black line in this figure. The red line shows the temperature calculated by multiplying 4.6% times the average of the existing temperature and the ending temperature of 28 oC at each 10-second interval. The 4.6% has to do with the conductivity per second of heat into the black object and other boundary conditions.
If you shine a light on a small black object, the rate of warming, the rate that heat flows, similarly decreases with decreasing difference in temperature forming an asymptotic curve shown by the black line in this figure. The red line shows the temperature calculated by multiplying 4.6% times the average of the existing temperature and the ending temperature of 28 oC at each 10-second interval. The 4.6% has to do with the conductivity per second of heat into the black object and other boundary conditions.
Similar asymptotic curves are observed for both warming and cooling of matter by conduction or by absorbing or emitting radiation. In this way, the flux of heat is observed to be based on temperature difference and is not additive as currently assumed by most climate scientists and most physicists. Heat and the flux of heat are both observed to be averative. This is an extremely important observation that is one of the primary reasons why greenhouse-warming theory is mistaken. Heat is what a body of matter must absorb to raise its temperature. Because temperature is averative, there is no physical way that heat could not be averative.
Heat is what a body of matter must absorb to raise its temperature. Because temperature is averative, there is no physical way that heat could not be averative.
But how does the warming body “know” the ending temperature of 28 oC and how, physically, does Nature determine an average? The answer is by resonance, also known as sympathetic vibration. Resonance is a fundamental physical property of oscillating systems. Remember that all bonds holding matter together are oscillating at all times.
The simplest example of resonance is a single frequency transmitted by a radio station. The radio program modulates a center frequency assigned by the government to prevent interference with other nearby stations. The radio transmitter causes oscillations of this modulated center frequency on the surface of the transmitting antenna. You tune your radio receiver to resonate at that specific center frequency. Resonance is the way two oscillators oscillating at the same frequency can, under the best of conditions, average their amplitudes of oscillation, effectively transferring amplitude of oscillation from the transmitter to the receiver. In this way, your radio receiver discriminates the center frequency of the radio station from all the other frequencies out there. It is the oscillatory nature of electromagnetic radiation that makes it possible for radiation, for heat, to be transmitted across air and space via resonance.
Resonance is observed to transfer amplitude of oscillation at a specific frequency of oscillation only from higher amplitude to lower amplitude, which Planck’s Law shows clearly is from higher temperature to lower temperature. Resonance occurs between one discrete molecular-scale oscillator on the surface of the emitting body and one discrete molecular-scale oscillator on the surface of the absorbing body. Radiant heat travels through air and space when resonance occurs simultaneously between different pairs of oscillators at each and every frequency of oscillation of all the molecular-scale oscillators on the surface of matter.
Resonance is observed to transfer amplitude of oscillation at a specific frequency of oscillation only from higher amplitude to lower amplitude, which Planck’s Law shows clearly is from higher temperature to lower temperature.
Resonance is all around us. You experience resonance most clearly when you push a child on a swing. If you push at exactly the same frequency of oscillation as the swing is swinging, the amplitude of the swing will increase. You tune radio and television receivers to resonate at whatever frequency your preferred station is transmitting. Your cellphone is tuned to resonate with the different frequencies of transmission and reception used at a local cell tower. Individual hair cells, called cilia, in the cochlea of your ears, resonate with sounds in air, sending signals to your brain, allowing you to hear very low frequencies typically from 20 to 20,000 cycles per second. Visible colors, which are frequencies of oscillation between 450 and 789 trillion cycles per second, resonate with cells in the cones of your eyes that send signals to your brain, allowing you to see ten million different colors. Visible light is visible because these are the natural frequencies of oscillation that the cells in your eyes oscillate at, given their physical size.
For centuries, scientists have argued whether light, in the form of electromagnetic radiation, travels through space as waves or as particles. Waves and particles, however, describe how the energies of motion of physical pieces of matter are visualized as traveling. But light is not physical matter and light cannot be observed until it interacts with physical matter. Light, electromagnetic radiation, is observed to be a broad continuum of frequencies of oscillation where amplitudes of oscillation are observed to travel simultaneously at each frequency of oscillation by resonance.
Light, electromagnetic radiation, is observed to be a broad continuum of frequencies of oscillation where amplitudes of oscillation are observed to travel simultaneously at each frequency of oscillation by resonance.
Conduction is resonance enabled by physical contact of molecules of matter. Radiation is resonance enabled by the interaction of what we think of as electric and magnetic fields via line of sight where what we think of as the velocity of light is proportional to the very short and constant time required for resonance to occur over any distance. Frequency of oscillation is observed not to change with distance, even over galactic distances, except for Doppler effects where the source and receiver are moving relative to each other. Similarly, amplitude of oscillation is not observed to change with distance. Thus, the Planck temperature of radiation from Sun is the same close to Sun as it is close to Earth. But the thermal effect of solar radiation is observed to decrease with the square of the distance. What appears to be happening is that the density of molecular-scale bonds on the surface of matter that resonate decreases with distance, so that the amplitude increase due to resonance must be shared with increasing numbers of bonds that are not resonating with bonds on the distant source. Resonance is explained in more detail in video 9.
Another key property of resonance is that the absorbing oscillator, in an undamped system, may oscillate at an amplitude much greater than the average between the emitter and the absorber. This explains why oxygen molecules are dissociated most efficiently at frequencies of oscillation close to 1237 terahertz and not at higher frequencies. This also explains the spectral lines of absorption by carbon dioxide discussed below that are thought to be the resonant frequencies of all the bonds that hold matter together. We still have a lot to learn about resonance, but we first have to realize how important it is for the flow of heat.
Nine Fundamental Mistakes in the Physics of Heat and in Greenhouse-Warming Theory
Numerous assumptions central to greenhouse-warming theory turn out to be mistaken.
(1) Radiant energy cannot be quantified by a single number of watts per square meter as assumed in physics for more than two centuries. Planck’s empirical law shows clearly that radiation from matter, as a function of temperature, consists of a broad spectrum or continuum of frequencies of oscillation. According to the well-accepted Planck-Einstein relation, each frequency has its own energy. Therefore, energy radiated by a body of matter is a continuum of energies where all energies coexist. It makes no physical sense to integrate across these frequencies or energies to calculate a single number of watts per square meter. This approach seems to have worked adequately for most engineering applications where temperature differences are small. It fails catastrophically, however, for large differences in temperatures such as between Sun at 5500 oC and Earth at 15 oC.
(2) Radiative forcings cannot be added together. Temperature and heat are not additive. They are averative because heat flows by resonance.
(3) Earth’s surface temperature is not proportional to the sum of all radiative forcings. Planck’s empirical law shows clearly that temperature in matter is the result of a broad continuum of frequencies of oscillation and associated continuum of amplitudes of oscillation. Planck’s empirical law shows what frequencies and amplitudes of oscillation must be occurring throughout a body of matter for that body to possess a specific temperature.
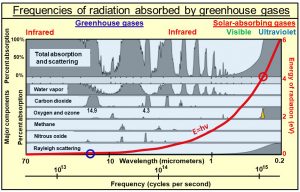 (4) Greenhouse gases absorb only certain limited bands of frequencies of radiation emitted by Earth as shown in this diagram. Water is, by far, the strongest absorber, especially at lower frequencies.
(4) Greenhouse gases absorb only certain limited bands of frequencies of radiation emitted by Earth as shown in this diagram. Water is, by far, the strongest absorber, especially at lower frequencies.
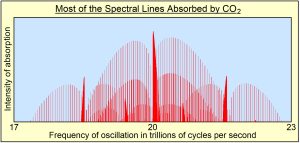 Within these narrow frequency bands, what is being absorbed is only narrow spectral lines of oscillation shown in red in the diagram on the left. These spectral lines turn out to be the resonant frequencies of all the overtones of oscillation, of all the normal modes of oscillation or all the bonds holding the molecules together. Radiant energy is absorbed into the bonds, which has no direct effect on the temperature of the gas. Temperature of a gas is proportional to the average kinetic energy of translation of all atoms and molecules making up air. One has to assume that the absorbed oscillatory energy is converted by collisions into translational kinetic energy, a process that does not appear to be efficient.
Within these narrow frequency bands, what is being absorbed is only narrow spectral lines of oscillation shown in red in the diagram on the left. These spectral lines turn out to be the resonant frequencies of all the overtones of oscillation, of all the normal modes of oscillation or all the bonds holding the molecules together. Radiant energy is absorbed into the bonds, which has no direct effect on the temperature of the gas. Temperature of a gas is proportional to the average kinetic energy of translation of all atoms and molecules making up air. One has to assume that the absorbed oscillatory energy is converted by collisions into translational kinetic energy, a process that does not appear to be efficient.
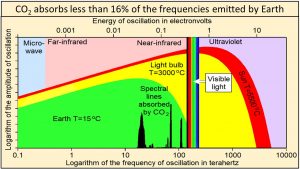 Ångström (1900) showed that “no more than about 16 percent of earth’s radiation can be absorbed by atmospheric carbon dioxide, and secondly, that the total absorption is very little dependent on the changes in the atmospheric carbon dioxide content, as long as it is not smaller than 0.2 of the existing value.” Extensive modern data agree that carbon dioxide absorbs less than 16% of the frequencies emitted by Earth shown by the vertical black lines of this plot of Planck’s empirical law where frequencies are plotted on a logarithmic x-axis. These vertical black lines show frequencies and relative amplitudes only. Their absolute amplitudes on this plot are arbitrary.
Ångström (1900) showed that “no more than about 16 percent of earth’s radiation can be absorbed by atmospheric carbon dioxide, and secondly, that the total absorption is very little dependent on the changes in the atmospheric carbon dioxide content, as long as it is not smaller than 0.2 of the existing value.” Extensive modern data agree that carbon dioxide absorbs less than 16% of the frequencies emitted by Earth shown by the vertical black lines of this plot of Planck’s empirical law where frequencies are plotted on a logarithmic x-axis. These vertical black lines show frequencies and relative amplitudes only. Their absolute amplitudes on this plot are arbitrary.
Temperature at Earth’s surface is the result of the broad continuum of oscillations shown in green. Absorbing less than 16% of the frequencies emitted by Earth cannot have much effect on the temperature of anything.
(5) Greenhouse gases can only reradiate what they absorb. A molecule of carbon dioxide cannot physically emit black-body radiation. It can only emit the spectral lines that it absorbed into its bonds. When these limited spectral lines are absorbed by any body of matter, they would have minuscule effect on temperature of that body of matter because they only make up a very small part of the continuum of frequencies whose amplitudes of oscillation must be increased in order to warm a body of matter.
(6) Greenhouse gases do not reradiate in every direction as assumed. Radiation by resonance is observed only to flow from a hotter body of matter to a cooler body of matter, which at the molecular-bond scale is from a higher amplitude of oscillation to a lower amplitude of oscillation at the same frequency.
(7) Carbon dioxide makes up only 0.04% of the atoms and molecules in air. Any increase in energy resulting from absorption by carbon dioxide, must be shared with 2500 other molecules and atoms.
(8) Ångström (1900) shows by two experiments that greenhouse-gas concentrations of carbon dioxide in the atmosphere have minimal effect on air temperature. My experiments show that increasing carbon dioxide concentrations by a factor of more than 23 has no measurable effect on air temperature. Experiments reported on Internet that claim to see greenhouse warming utilize heat sources that are much, much hotter than Earth. More information at JustProveCO2.com.
(9) The thermal effects of radiation are not about amount of radiation absorbed, as currently assumed, they are about the temperature of the emitting body and the difference in temperature between the emitting and the absorbing bodies as explained above.
Greenhouse warming theory depends on at least nine assumptions that appear to be mistaken. Greenhouse warming theory has never been shown to be physically possible by experiment, a cornerstone of the scientific method. Greenhouse warming theory is rapidly becoming the most expensive mistake ever made in the history of science, economically, politically, and environmentally as explained in detail in sixteen short videos found at WhyClimateChanges.com/most-expensive-mistake/. Video 1 is an introduction. Videos 2 through 6 describe evidence for global warming and for the role of humans and of volcanic eruptions in causing observed climate change. Videos 7 through 10 explain what is mistaken concerning greenhouse-warming theory and why this theory is physically impossible. Videos 11 through 16 discuss issues related to setting informed public policy concerning climate change. A separate video, A Most Unexpected Revolution in the Physics of Heat, explains current problems with the physics of heat.
Click here for a 20-page scientific paper A Most Inconvenient Reality — Greenhouse Gases Cannot Physically Explain Observed Global Warming submitted to the Journal of Geophysical Research on May 28, 2018, that describes these issues in more detail. This file includes the editor’s email rejecting the paper without review and my response.
Greenhouse warming theory is rapidly becoming the most expensive mistake ever made in the history of science, economically, politically, and environmentally.
The Crisis in Climate Science
Climate science is in a state of crisis because climate scientists, enamored with their broad consensus regarding greenhouse-warming theory, refuse to face the remarkably clear physical reality that greenhouse-warming theory is not even physically possible. What has happened to scientific objectivity? Politics requires consensus, but science requires debate. As Michael Crichton explained: “In science consensus is irrelevant. What is relevant is reproducible results. The greatest scientists in history are great precisely because they broke with the consensus. There is no such thing as consensus science. If it’s consensus, it isn’t science. If it’s science, it isn’t consensus. Period.”
In science consensus is irrelevant. What is relevant is reproducible results. The greatest scientists in history are great precisely because they broke with the consensus. There is no such thing as consensus science. If it’s consensus, it isn’t science. If it’s science, it isn’t consensus. Period.” Michael Crichton
A Global Challenge
All of the issues discussed on this web page were known to me by 2015. Since then I have tried to get my fellow scientists to stop and think. I have written many papers, which when submitted for publication were not even sent out for review. I have sought interaction with many leading climate scientists and many climate skeptics. I have gone to great lengths, as explained in video 12, to try to get people to recognize that greenhouse-warming theory appears to be mistaken.
On this web page, I have tried to explain the issues as cogently at I can. I challenge anyone in the world to find any serious problem on this web page that could change the clear conclusion that greenhouse-warming theory is not only mistaken, it is not even physically possible. Please ask anyone defending greenhouse-warming theory to find the error in the observations reported on this web page. I issued this challenge in a major way on November 4, 2019, described at WhyClimateChanges.com/the-global-challenge/.
I challenge anyone in the world to find any serious problem on this web page that could change the clear conclusion that greenhouse-warming theory is not only mistaken, it is not even physically possible.” Dr. Peter L. Ward
If you have any good ideas pro or con, please join the scientific discussion at groups.google.com/d/forum/co2impossible which follows below.
Dr. Peter Langdon Ward (bio)